Keywords
Gamete; GIFT; Lineage; Tilapia
Introduction
The tilapia culture has witnessed a considerable growth in recThe tilapia culture has witnessed a considerable growth in recent years, driven mainly by growth performance of species and their acceptance in market, putting them among the most produced in the world (Yasui, G.S., et al. 2007).
According to Moreira et al. (2007), rearing of tilapia in Brazil had the initial introduction of a breeding tilapia rendalli in the 1950s, followed by a new introduction of Nile tilapia in 1972. World production of tilapia has focused on three species: Oreochromis niloticus, Oreochromis mossambicus and Oreoclhromis aureus (Coward, K., 2000). Since the dominating niloticus 44% of the total produced in the world of tilapia (Rana, k.J. 1990).
The sexual maturity of this species is related to the age and size and can be tilapia although easy to grow fish; this may present some constraints such as the early breeding, which occurs from four months and 12 months (Proença E.C.M., 1994). So in order to produce genetically modified animals, both in the reproductive and economically, were developed several programs for genetic improvement of tilapia. Among them, GIFT Genetic Improvement of Farmed Tilapia, which consists of an inventory developed through combinations of species, producing a strain genetically improved, with better weight gain and feed conversion than the other strains of tilapia (Joen, G.H.M. 2005).
Despite its good GIFT reproductive performance are scarce in the literature that reports the reproduction of this species. According to Coward and Bromage (Coward, K., 2000), advances in understanding the reproduction of tilapia are essential to overcome the restrictions of their culture, and promote ease of handling and experimentation.
This study aimed to obtain information on the reproductive performance of tilapia GIFT, and compare the influence of the weight of breeding fish in the quality of gametes.
Material and Methods
Selection of Breeding
The selection of breeding occurred in Fish Aquasport Station located in Br 491 Km 158 at Alfenas city in Minas Gerais State- Brazil. Thirty healthy breeding females and males weighing between 250 and 500 g (light animals) and thirty breeding females and males ranging in weight from 600 g to 1000 g (heavy animals) were used. The selection of males was performed by preliminary evaluation of urogenital papilla and elimination of semen by manual compression of the coelomic cavity. In turn, in females we had used abdominal pressure next to the urogenital papilla to allow the oocytes output. After selection, the animals were placed in hapas and then separated by weight and sex in reproduction tanks and fed with extruded feed for a period of twenty days. No applications of reproduction induce hormones was used in this work.
Semen Analysis
Collection and Semen Volume
Obtaining semen consisted of light massage on the ventral region, and semen was collected in identified microtubule, with a capacity of 2 mL and free of any contaminants (urine, water or blood). The semen quality was assessed according to the following parameters.
Sperm Concentration
The sperm concentration was carried out individually. 1μl of semen was diluted in 990μl of formaldehyde. We used the method of sperm count in a Neubauer chamber hematology. Sperm cells present were counted in ten fields of the chamber (Streit, J.R. D.P., et al. 2003, Wirtz, S., 2006). The sperm concentration was calculated similarly recommended by CBRA (1998).
Sperm Morphology
To analysis of sperm morphology were counted 200 sperm cells from each animal. The sperm abnormalities classification was based on the damage proposed y Paulino et al. (Paulino, M.S., 2011) and (Miliorini et al. 2011).
Sperm Motility
Under optical microscope slide 26x76 mm, a drop of semen was diluted in 0.03 mL distilled water then the slide was brought to phase-contrast microscope 40x, by assessing the sperm motility percentage and duration of the swimming ability of sperm. The sperm motility percentage was used a score of 0-100% and the time duration of the motility was analyzed over time in seconds of the swimming capacity of spermatozoa (Matavelli, M., et al. 2007).
Collection and Analysis of oocytes
Through massage on the ventral region, the oocytes were collected in petri dishes and investigated the total weight of spawning, shape and color of oocytes and oocyte diameter.
To measure the diameter of the oocyte, a sample of the oocytes was determined in solution by Gilson (50mL of 60% alcohol. 440cc of distilled water, 7 mL of nitric acid, 10 g of mercuric chloride - HgCl2 and 9 ml glacial acetic acid) for 30 minutes, the oocytes were measured (μm) with the aid of ocular micrometer (10x) under an optical microscope (40x), Pereira et al. (Pereira, G.J.M., 2009).
Analysis of the Gametes Ultraestrutual
Morphology of the micropyle and Blocking Polyspermy and Morphometry of Sperm
We analyzed the morphometric measurements of sperm (length and width of head, length of the middle piece and flagellum). Oocytes were analyzed in the size of the ostium and vestibule of micropyle and time of opening of the micropyle. Both gametes were fixed in Karnovsk solution (2.5% glutaraldehyde, 2.0% paraformaldehyde in sodium cacodylate buffer 0.05 M, pH 7.2, calcium chloride, 0.001 M) for further analysis. For analysis of the opening time of the lock and micropyle polyspermy, gametes samples of six breeder males and females were mixed individually, 1μl of sperm into 2 g oocytes and hydrated with water tank at a time of 0 seconds to 30 seconds.
The samples were diluted in 1% osmium tetroxide for 4 hours at room temperature and subsequent dehydration through a series of increasing acetone (25%, 50%, 75%, 90% and 100%). Prior to evaluation by scanning electron microscopy, the samples were dehydrated with the aid of apparatus CPD030 critical, and the samples coated with gold under vacuum evaporator SCD 050, using methods adapted for Psenicka et al. 2009.
Results and Discussion
Semen Analysis
Sperm Concentration and Seminal Volume
The fish weight did not influence (P>0.05) semen volume, which averaged 0.30 ± 0.07 ml, and had effect on sperm concentration (P<0.05), whose values for heavy animals were 2.44 ± 0.41×109 sperm/ml and light animals were 1.66 ± 0.14×109 sperm/ml. Possibly the temperature factor may have interfered in the seminal volume of species studied, since the collection was held in June, when environmental temperatures are reduced.
However, the temperature can be influenced in the least amount of sperm per ml, in comparison with other species. According to Godinho et al. (2007) that variation in sperm concentration can be explained by several factors, such as provision of the individual, the particular species, collection method and season of year, being the time for collecting a variable that can limit the production of sperm cells (Borges, A., et al. 2005).
Similar studies with Oreochromis niloticus confirm the hypothesis of temperature influence the amount of sperm per ml. Matavelli et al. (2007), to perform collection in August, showed similar results to the results of our study, which found in semen volume of 0.40 ml and the concentration of 2.63×109 ml-1.
Sperm Morphology
The weight influenced major and minor sperm abnormalities (P<0.05). In heavy animals, anomalies tail tucked, head and middle piece isolated degenerate, were higher with averages of 5.9 ± 0.6, 23.6 ± 1.1 and 18.8 ± 2.3 respectively compared to light animals with averages of 2.6 ± 0.7, 5.5 ± 0.4 and 13.1 ± 3.5, respectively. On the other hand the anomaly of the head showed degenerate was higher in light animals, with averages of 2.8 ± 0.4 in relation to heavy animals, whose average was 1.0 ± 0.4. Figures 1, 2 and 3 denote the major and minor abnormalities in animals heavy and light.
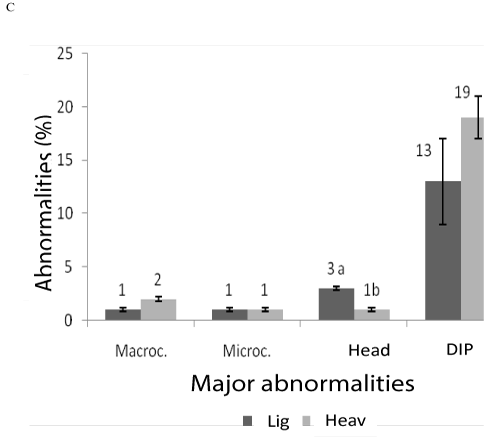
Figure 1: Sperm abnormalities in tilapia GIyF T heavy and light. Macrocephaly, Microcephaly, head degenerate and degenerate intermediate piece (PID).
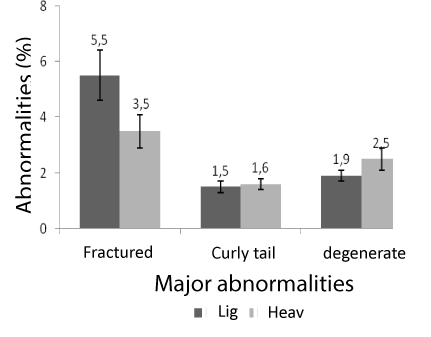
Figure 2: Sperm abnormalities in tilapia GIFT heavy and light. Fractured tail, curly tail and tail degenerate.
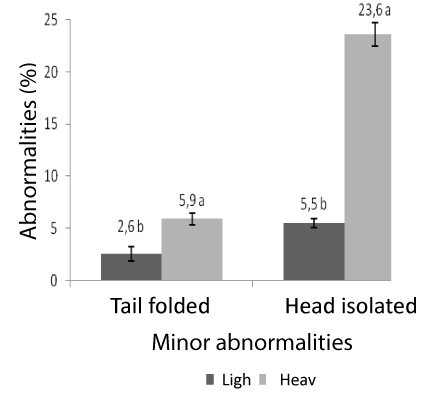
Figure 3: Sperm abnormalities in tilapia GyI FT heavy and light. Head isolated and tail folded.
This value is within the acceptable limits for use to fertilization according to Miliorini et al. (2011) who reported that the critical percentage of total sperm abnormalities to be used for artificial fertilization is below 50%, given that this technique involves a high ratio of sperm: oocytes in a controlled environment.
According Cosson, J. (1999) the increase in abnormal sperm causes a decrease in sperm motility, unlike in the present study despite the high rate of abnormal sperm head, middle piece and tail, these did not affect sperm motility in animals and heavy, since both showed high rate of mobile sperm was over 80%. However rate of normal sperm were lower when compared to studies of other tilapia strains. Matavelli et al. (2007) found that sperm from Oreochromis niloticus showed 16.28% of normal spermatozoa.
The index of more pronounced morphological abnormalities in animals weighed can be explained by the age of the animals, since the seminal production can be influenced by several factors: size of the individual, Mendes (2003), the reproductive age, Bastard et al. (2004), successive collections seminal Kavamoto et al. (1999) and season (Borges, A., 2005).
Sperm Motility
Sperm motility of heavy animals was similar (P>0.05) to the light animals, being, respectively, 86 ± 6% and 96 ± 1%. The weight did not influence the duration of motility, with 153 ± 24 seconds for heavy animals and 143 ± 26 seconds to light animals. These data were higher than those reported by Matavelli et al., who found 81.08% of motility in O. niloticus. Although the authors have used vitamin C supplementation, there was no interference of this addition in the motile parameters.In many species of fish, sperm motility is triggered after contact with a hypotonic and motility lasts too fast. Thus, the use of salt solutions prolongs the ability of the sperm fish swimming. As an example, the motility of Oreochromis niloticus may be extended by up to 15 minutes if activated with NaCl or KCl, Mochida et al. (1999). Distilled water, in turn, was used in this study in order to verify the effectiveness of sperm motility and not the efficiency of saline. While not include activating solutions, sperm motility showed satisfactory rates of these species, demonstrating that the animals were able to reproduce, regardless of weight and time of semen collection.
Analysis of oocytes
The weight of the animals did not influence (P>0.05) on the total weight of spawning, which averaged 58 ± 7 g and 60 ± 8 g in animals light and heavy, respectively, and the yellow yolk oocytes showed irregularly shaped.
Lowe and McConell (1955) observed that the size of the egg tilapia is species-specific, although there is evidence to suggest that larger eggs are produced by the larger fish (Trewavas E. (1982).Conversely, the diameter of oocytes from GIFT tilapia verified in this study did not influence (P>0.05) by the weight of the animals, being 1.31 ± 0.04 g for light animals and 1.27 ± 0.02 for heavy animals, which allows to infer that age oocyte volume does not interfere with the species.
The evaluation of the diameter is a fast method for confirmation of the maturity of the fish, Vazzoler (1981), and is therefore widely used for the selection of breeding females (Pereira, G.J.M., 2009). In addition to decrease problems associated with the fish farm as low fecundity and asynchronous spawning (Coward, K., 2000).
Species of tilapia have average oocytes diameters greater than those presented in our work, usually from 1.5 mm to 2 mm in diameter (Coward, K., 2000). But in native species such as Prochilodus lineatus, induced with hormones the media of the oocyte diameter ranging from 1.45 mm Vazzoler (1996) and 1.36 mm (Pereira, G.J.M., 2009).
The variation of egg size has also been observed in several marine teleosts, although the mechanisms that influence this variation remain unclear (Coward, K., 2000). However there are studies that report that the size of the oocyte in several teleosts can be strongly influenced by the nutritional status of the female (Bromage, N.R., 1992). Studies by Gunasakasa and Lam (1997), show that the reduced protein content in the diet can significantly decrease the proportion of the vitellogenin oocyte/ovary. But in a study made with 20-35% in the diet of Oreochromis niloticus feeding increased fertility despite the size of the oocyte have remained unchanged.
Other studies also report that the protein-based diet can influence the growth and maturation of oocytes (Rosanthi, M., 1995). However, the reproductive physiology of tilapia species is complex and requires further investigation (Coward, K., 2000).
According to these authors, there are several factors that may be involved in oocyte size, including growth factors that regulate the receptor for vitellogenin. They may play a critical role in oocyte growth.
We can also assume that the low egg size of tilapia may have been influenced by collection time, since the time of spawning occurred in low temperature.
Ultrastructural Analysis of the Gametes
Morphology of the Micropyle, Blocking Polyspermy and Morphometry of Sperm.
The micropyle tilapia GIFT analyzed after being presented a funnel-shaped depression, with vocal arrangements around the micropylar region. This tapered shape has also been reported in European catfish (Silurus glanis) (Kudo, S., 1994), piracanjuba (Brycon orbignyanus) (Ganeco, L.N., 2003) and curimatã-pacu (Prochilodus marggravii) (Rizzo, E., 2003).
According to Riehl (1993) the arrangement of pores or channels in the zona radiata and the morphology of the micropyle are characteristic of specific species. Oocytes opening micropyle started at the time of hydration, and thirty minutes after the entry of sperm, a protrusion was observed in micropylar channel and starts the closure micropyle.
According Iwamatsu et al. (2000) the link between sperm fertilizing the egg surface causes a series of reactions that prevent supernumerary sperm entering the oocyte. This phenomenon is called the blocking polyspermy and is very important in normal development of monoespermatic eggs. In Brazil native species as Brycon orbignyanus (Piracanjuba), this block is characterized by the formation of cone fertilization (Ganeco, L.N., 2001). In other species like Silurus glanis (catfish) after the fusion of sperm in the micropylar opening is a cytoplasmic eminence that disappears after fertilization Kudo et al. (1994). According to these authors, this bulge is a specialized structure for the entry of sperm and its termination occurs after the fusion of gametes.
The sperm of tilapia GIFT showed simple organization: head without acrosome, middle piece and tail. The head showed ovoid form with measurements of length and width of 1.66 ± 0.02 μm and 1.5 ± 0.01 μm, respectively. The middle piece is presenting cylindrical length and width of 0.77 ± 0.03 μm and 0.98 ± 0.03 μm, respectively. The flagellum is unique and presents averages of 20 ± 0.28 μm and the total length of spermatozoa was 23.7 ± 0.31 micrometers.
These characteristics are presented in marine fish sperm, besides presenting different morphology as the acrosome inserted into the elongated head and middle piece short. Alavi et al. (2009) studying Northern pike, Esox lucius, found that the sperm head, middle piece, and flagellum length measurements were 1.32, 1.17, 32.0 and 34.4 μm, respectively.
In the sturgeon species as Acipenser sinensis, the values are 4.48, 3.3 and 37.8 μm respectively (Wei, Q., 2007). In other ruthenus as Acipenser, the data were 4.17, 0.97 and 42.47 μm for the length of the head, middle piece and flagellum respectively (Borges, A., et al. 2005). Acipenser Berii already in the intermediate part is relatively large in comparison with other species of sturgeon (1.09 ± 0.42 μm) and exhibits a long flagellum (44.75 ± 4.93 μm) (Psenicka, B.Y.M., 2007).
According to Jamieson (1991) and Gwo et al. (2000) sperm of fish with external fertilization have sperm head shapes spherical or round intermediate small numbers.
These morphological structure of sperm found in mariner fish and elongated head, middle piece and flagellum little too long characterized the differences between the structure of spermatozoa of freshwater fish and marine species, possibly influenced by the type of fertilization strategy. However, this does not seem to be the rule, as in other marine teleost Cottus gobio as the morphometric measurements and morphology can differentiate and present ovoid head and middle piece cylindrical with lengths of 2.25 ± 0.12 μm and 1.98 ± 0.12 μm, respectively (Lahnsteiner, F., 2008). In "African catfish," Mansour et al. (2002) the head size has been reported showing sizes (1.55 ± 0.10 mm in length and 1.36 ± 0.4 mm in width) and flagella (37.8 ± 1.3 mm in length) in however despite the intermediate part showing the cylindrical shape, are smaller showing size of 0.5 ± 0.06 mm in length.
In Brazilian native species sperm have very similar characteristics. In Salminus brasiliensis, Brycon microlepis and Brycon orbignayanus intermediate piece is thin and long and features asymmetrical measures 3.0 micrometre (Verissimo, S.R., 2006).
However, despite the GIFT tilapia structures show similarities in their sperm compared to native species of freshwater, it differs in the aspect of the format and length of the head and middle piece. The fact that the intermediate parts of the total spawning species were higher when compared with species spawning plot can be related to the type of fertilization.
Conclusion
Taking into account the parameters studied, tilapia breeding GIFT between 250 and 1.000 g can be used in commercial breeding herds and comparable to the traditional lineages.
Acknowledgment
CAPES, CNPq and Fapemig for financial support, and Pisciculture Aquaporto.
9103
References
- Yasui, G.S., Santos, L.C., Shimoda, E., RibeiroFilho, O.P., Calado, L.L., et al. (2007)Masculinização de Trêslinhagens de tilápias do Niloutilizando o andrógenosintético 17-α-metil-testosterona. [Portuguese] Zootecnia Trop, 25, 307-331
- nMoreira, A.A., Hilsdorf, A.W.S,, Silva, J.V., Souza, V.R., Ferreira, E.B.(2007)VariabilidadeGenética de duasvariedades de tilápianilóticapormeio de marcadoresmicrosatelite. PesqAgropecBras, 42, 521-526
- nCoward, K., Bromage, N.R. (2000) Reproductive physioloy of female tilápiabroodstock. Rev Fish Biol Fisher, 10, 1-25
- nRana, k.J.(1990) Influence of incubation temperature on Oreochromisniloticus (L.) eggs and fry II. Survival, growth and feeding of fry developing solely on their yolk reserves. Aquaculture, 87,183-195
- nProença, E.C.M., Bittencourt, P.R.L. (1994) Manual de piscicultura tropical. [Portuguese] Brasília IBAMA
- nJoen, G.H.M.(2005) Genetic changes during mass selection for growth in Nile tilapia Orechromisniloticus, assessed by microsatelites. Aquaculture Research, 36, 69-78
- nStreit, J.R. D.P., Moraes, G.V., Ribeiro, R.P., Caçador, W.C., Sakaguti, E.S. et al.(2003). Estudocomparativo da indução hormonal da espermiaçãoempiavuçu (Leporinusmacrocephalus) com extrato de hipófise de frango, coelho e carpa.ActaSciAnimSci, 25, 261-266
- nWirtz, S., Steinmann, P. (2006) Sperm characteristics in perch Percafluviatilis. J Fish Biol, 68, 1896-1902
- nColégioBrasileiro de Reprodução Animal -CBRA.Manual paraexamesandrológicos e avaliação de sêmen animal. [Portuguese] 2. ed. Belo Horizonte CBRA, 1998
- nPaulino, M.S., Miliorini, A.B., Murgas, L.D.S., Lima, F.S.M., Felizardo, V.O (2011). Desempenhoreprodutivo do pacu,piracanjubaecurimba,induzidos com extrato de buserelina.[Portuguese]Boletim do Instituto de Pesca, 37, 39-45
- nMiliorini, A.B., Murgas, L.D.S., Rosa, L.V.E., Oberlender, G., Pereira, G.J.N. et al. (2011) Amorfhological classification proposal for curimba (Prochiloduslineatus) sperm damages after cryopreservation. Aquac Res, 41, 1-11
- nMatavelli, M., Moraes, G.V., Streit, Jr. D.P., Varg, L.D.M., Sakagutio, E.S. et al .(2007) Avaliação da qualidade do sêmen de tilápia do Nilo (Oreochromisniloticus), linhagemchitraladasuplementada com diferentesconcentrações de vitamina C. [Portuguese] Boletim do Instituto de Pesca , 33, 1-7
- nPereira, G.J.M., Murgas, L.D.S., Silva, J.M.A., Miliorini, A.B., Logato, P.V.R. (2009) Indução da desova de curimba (Prochiloduslineatus) utilizando ECG e EBHC. [Portuguese] Revista Ceres, 156, 156-160
- nPsenicka, M., Vancova, M., Koubek, P.M., Tesitel, J., Linhart, O. (2009) Fine structure and morphology of sterlet (Acipenserruthenus L. 1758) spermatozoa and acrosin localization. AnimReprodSci, 111, 3-16
- nGodinho, H.P.(2007) Estratégiasreprodutivas de peixesaplicadas á aquicultura: bases para o desenvolvimento de tecnologias de produção. Rev. Bras. Reprod. Animal ,31, 351-360
- nBorges, A., Siqueira, D.R., Jurinitz, D.F., Zanini, R., Amaral, F. et al. (2005) Wassermann GF.Biochemical composition of seminal plasma and annual variations in sêmencharacteistics of jundiá, Rhamdiaquelen (Quoy and Gaimard, Pimelodidae). Fish PhysiolBiochem, 31, 45-53
- nCosson, J. (1999) Frenetic activation of fish spermatozoa flagella entails short-term motility, portinding their precocious decadence. J fish Biol, 76, 240-279
- nMendes, H.F., Casatti, L., Ferreira, K.M. (2003) Aquatic macrophytes as feeding site for small fishes in the Rosana reservoir, Paranapanema River, southeastern Brazil. Braz J Biol, 63, 213-222
- nBastardo, H., Guedez, C., León, M. (2004) Características Del sêmen de truchaarco-íris de diferentesedades, bajocondiciones de cultivoem Mérida, Venezuela.Zootec Trop, 22, 277-288
- nKavamoto, E.T., Barnabe, V.H.,Campos, B.E.S., Andrade-Talmelli, E.F(1999) Anormalidadesmorfológicasnosespermatozoides do curimbá, Prochilodusscrofa(Steindachner, 1881) (Osteichthyes, Characiformes, Prochilodontidae). [Portuguese] Boletim do Instituto de Pesca, 25, 61-66
- nMochida, K., Tomoko, K., Matsubara, T., Adachi, S., Yamauchi, K. (1999) A high molecular weight glycoprotein in seminal plasma is a sperm immobilizing factor in the teleost Nile tilápia, Oreochromisniloticus. Develop Growth Differ, 41, 619-627
- nLowe-McConnell RH. (1955) The fecundity of Tilapia species. East AfrAgricJ, 21, 45-52
- nTrewavas E. (1982) Generic groupings of Tilapiini used in aquaculture. Aquaculture,27,79-81
- nVazzoler AEAM Manual de métodos par estudosbiológicossobrepopulações de peixes: crescimento e reprodução. [Portuguese] Brasília CNPq, 1981
- nVazzoler AEM. Biologia da reprodução de peixesteleósteos: teoria e prática. Maringá EDUEM/Nupélia, 1996
- nBromage, N.R., Jones, J., Randal, C., Thrush, M., Davies, B.(1992) Broodstock management, fecundity, egg quality and timing of egg production in the rainbow trout (Oncorhynchusmykiss). Aquaculture, 100, 141-166
- nGunasekera, R.M., Lam, T.J. (1997) Influence of dietary protein level on ovarian recrudesence in Nile tilápia, Oreochromisniloticus. Aquaculture, 149, 57-69
- nRosanthi, M., Gunasekara, K.F., Stim, T.J. (1995) Effect of dietary protein level on puberty oovcyte growth and egg chemical composition in the tilápia, Oreochromisniloticus (L).Aquaculture, 134, 169-183
- nKudo, S., Linhard, O., Billard, R. (1994) Ultastructural studies of sperm penetration in the egg of the European catfish, Silurusglanis. Aquat Living Resour, 7, 93-98
- nGaneco, L.N., Nakagi, L.S.O., Godinho, H.P. (2003) Morfologia da micrópila e da superfíce dos ovócitos de piracanjuba, Bryconorbignyanus (Osteichthyes, Characidae), sob microscopiaeletrônica de varredura. ActaScientBiolSci, 25, 227-231
- nRizzo, E., Godinho, H.P., Sato, Y. (2003) Short-term storage of oocytes from the neotropical teleost fihProchilodusmarggravii. Theriogenology, 60, 1059-1070
- nRiehl, R. (1993) Surface morphology and micropyle as a tool for identifying fish eggs by scanning electron microscopy.MicroscAnalyis, 4, 29-31
- nIwamatsu T. Fertilization in fishes. In: Tarín, J.J.;Cano A. (Ed). Fertilization inprotozoa and Metazoa Animal. Heidelberg Springer-Verlag Berling, 2000
- nGaneco, L.N., Nakaghi, L.S.O., Urbinati, E.C., Dumont-Neto, R., Vasques, L.H.(2001) Análisemorfológica do desenvolvimentoovocitário de Piracanjuba, Bryconorbignyanus, durante o cicloreprodutivo. [Portuguese] Boletim do Instituto de Pesca, 27, 131-138
- nAlavi, S.M., Rondina, M., Viveiros, A.T.M., Cosson, J., Gela, D. et al (2009) Effects of osmolity on sperm morphology, motility and flagellar wave parameters in Northen pike (Esoxlucius L.). Theriogenology, 72,32-43
- nWei, Q., Psenicka, P., Alavi, A.S.M., Shen, L.,Liu, J. et al.(2007) Ultrastructure and morphology of spermstozoa in Chinese sturgeon (Acipensersinensis Gray 1835) using scanning and transmission electronmicroscopy. Theriogenology, 67, 1269-1278
- nPsenicka, B.Y.M., HadiAlavi, R., Gela, D., Nebesarova, J., Linhart, O. (2007).Morphology and ultrastructure of Siberian sturgeon using scanning and transmission electron microscopy. Biol Cell, 99, 103-115
- nJamieson, B.G.M. (1991) Fish evolution and systematics: evidence from spermatozoa.Cambridge University
- nGwo, J.C. ( 2000) Cryopreservation of sperm of some marine fishes. In: Tiersch TR,Mazik PM (eds.). Cryopreservation in aquatic species.Baton Rouge, Louisiana, USA World Aquaculture Society
- nLahnsteiner, F., Patzner, R.A.S., Alavi, J.J., Cosson, K. (2008) Sperm morphology and ultrastructure in fish. In: Coward and G. Rafiee (Eds). Fish Spermatology. Oxford Alpha Science Ltd
- nMansour, N., Lahnsteiner, F., Patzner, R.A. (2002)The spermatozoon of the African catfish: fine structure, motiity, viability and its behaviour in seminal vesicle secretion. J fish Biol, 60, 545-560
- nVerissimo, S.R., Gusmão-pompiani, P., Vicentini, C.A., Quagio-Grassiotto,I. (2006) Spermiogenesis and spermatozoa ultrastructure in Salminus and Brycon, two primitive genera in Characidae (Teleostei: Ostariophysi: Characiformes). ActaZool ,87, 305-313
- nFerreira, A.A., Nuner, A.P.O., Luz, R.K., Tataje, D.A.R. (2001) Avaliaçãoqualitativa e quantitativa do semen de jundiáRhandiaquelen. [Portuguese] Boletim do Instituto de pesca, 27, 57-60
- nGodinho, H.P., Amorin, M.C., Peixoto, T.D. (2003) Criopreservação do sêmen de tilápia do NiloOreochromisniloticus, varidadechitralada: crioprotetores, soluçõesativadoras e refrigeradorcriogênico. R Bras Zootec, 32, 1537-1543.









