Keywords
Aslantas Dam Lake, Rotifera, Chlorophyll a
Introduction
It is a well-established fact that phytoplankton is the base of food chain pyramid in the aquatic environments such as lakes. Zooplankton, following phytoplankton, is the second most important ring of the pyramid of aquatic ecosystems for energy conversion and as food resources. Therefore the importance of zooplankton for fish production and fisheries is obvious. At the same time, many scientists indicated that certain species of rotifers are indicators showing water quality, pollution and eutrophication. (Berzins, 1987; Mikschi, 1993; Saksena, 1987; Sharma, 1983).
This paper presents an analysis of rotifer abundance in Aslantas Dam Lake which is one of the most important freshwater resources of south eastern Mediterranean region of Turkey. There have been no previous rotifer studies undertaken for this lake. The dam was established over the River Ceyhan at 45 km up from Mediterranean Sea for flood prevention, irrigation and hydro electric energy production in 1985. The dam is situated 146 m above the sea level and covers an area of 6.050 ha. Maximum measurements of width and length are 6 km and 16 km, respectively, and the deepest place in the lake measures 93 m (DSI, 1966).
Besides agriculture, fish such as Cyprinus carpio, Silurus glanis, Capoeta sp., Barbus sp., Leiciscus cephalus and Alburnus orentis are providing an important economical benefit for the local people although the dam was built for irrigation purposes DSI, 2000).
Materials and Methods
The abundance of rotifer fauna in Aslantas Dam Lake was observed between March 2001 and April 2002 using 5 stations for sampling (Figure 1). Sampling was made in monthly intervals except December due to bad weather conditions. Besides, dissolved oxygen analyses of water in April and May were not recorded caused by oxygen meter malfunction.
Figure 1. Stations in Aslantas Dam Lake
Rotifer samples were collected using horizontal plankton net with 60 μm mesh, 1 m length and 30 cm in diameter, and standard Hydrobiyos brand vertical plankton net with 60 μm mesh, and the samples were collected from the surface and different depths in all stations. Water sampling was made using Nansen bottle and the samples were taken from the surface in all stations except Station 1 and Station 2, where sampling was extended to 50 m depth by taking samples from 0.0- 1.0-2.5-5.0-7.5-10.0-15.0-20.0-30.0-40.0-50.0 m depths.
Parameters such as dissolved oxygen (DO), temperature, pH, light penetration measurements were recorded during sampling time at study area. Additionally, the depth of photic zone (light penetration zone) at stations 1 and 2 was determined by multiplying the depth of Secchi disk with 2.7 (Cole, 1983; Moss, 1988). Chlorophyll-a was analyzed using the method described by APHA, (1995).
The samples, collected with nets and preserved in 4% formaldehyde, were used for qualitative analyses (classification) of rotifer (Dussart, 1969; Voigt and Koste, 1978; Stemberger, 1979; Tsalolikhin, 1994; Tsalolikhin, 1995). Quantitative analyses was carried out following filtration of 5 L water trough 60 μm meshes and preserving the results in 4% formaldehyde.
Results and Discussion
The analyses and observations revealed the following results for physico-chemical parameters shown in Table 1.
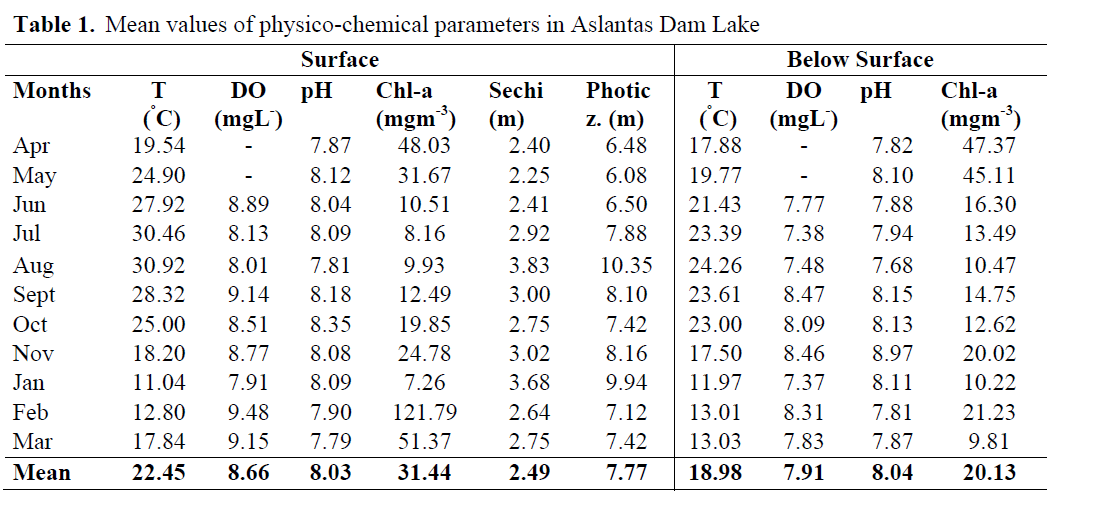
Table 1. Mean values of physico-chemical parameters in Aslantas Dam Lake
Maximum temperatures were measured in August as 30.92ºC, whereas minimum temperatures were reached in January as 11.04ºC.
The level of DO was maximum (9.48 mgL-1) in February and minimum (7.37 mgL-1) in January. The highest pH was 8.97 in November while it was the lowest with 7.68 in August.
Chlorophyll a at surface reached the highest level with 121,79 mgm-3 in February, and the level found out to be the lowest in January with 7.26 mgm-3.
The last parameter, light penetration found to be maximum in August (3.83 m) and minimum in May (2.25 m). Consequently, the range of photic zone was determined to be the largest in August it was determined that the range of photic zone extended to maximum 10.35 m in August and minimum 6.08m in May. Additionally, vertical average values physico-chemical parameters was presented.
The maximum values of the parameters were observed in different depth i.e. temperature (22.40oC) at 1 m, DO (8.58 mgL-1) at surface, pH (8.07) at 5 m, and chlorophyll a (34.51 mgm-3) at 7.5 m. However the minimum values were observed at the depths below 30 m, It was 12.10oC at 35 m, 6.01 mgL- 1 at 32 m, 7.59 at 50 m and 2.22 mgm-3 at 32 m, respectively (Table 2).
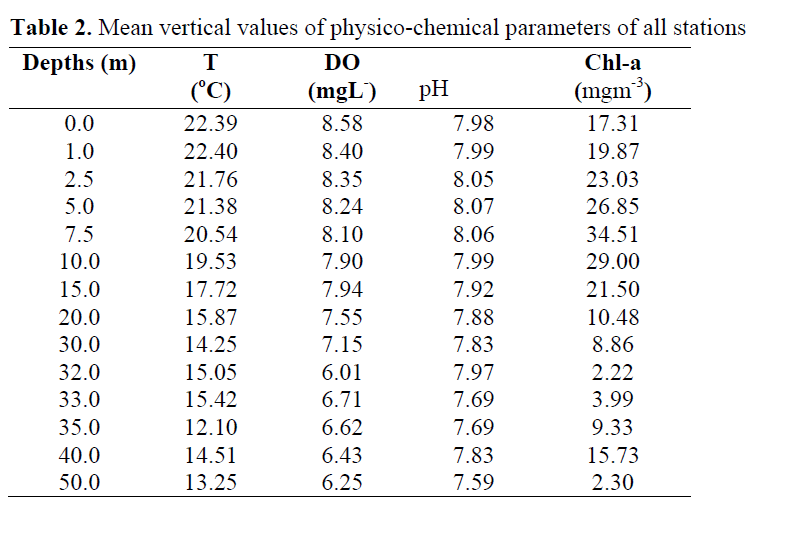
Table 2. Mean vertical values of physico-chemical parameters of all stations
Taxonomy
As result of this study, it was determined that rotifers in Aslantas Dam Lake are represented with 33 species and 2 subspecies (Table 3). They belong to 15 families in which the number of genus is 21. Determined fauna is listed below according to classification by Wallace and Snell (1991).
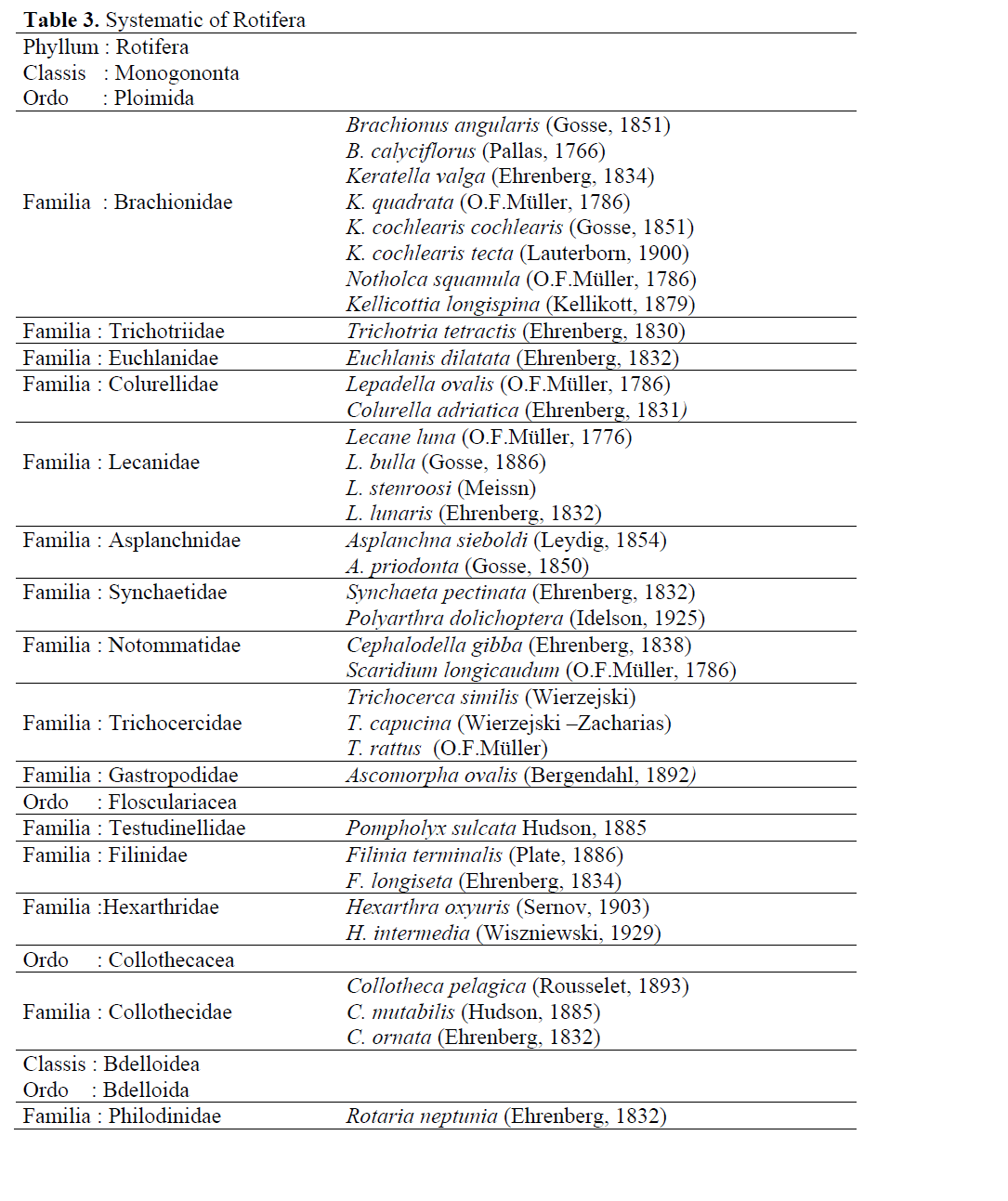
Table 3. Systematic of Rotifera
Monthly distribution
The monthly presence of determined rotifers at surface and below surface was tabulated in Table 4.
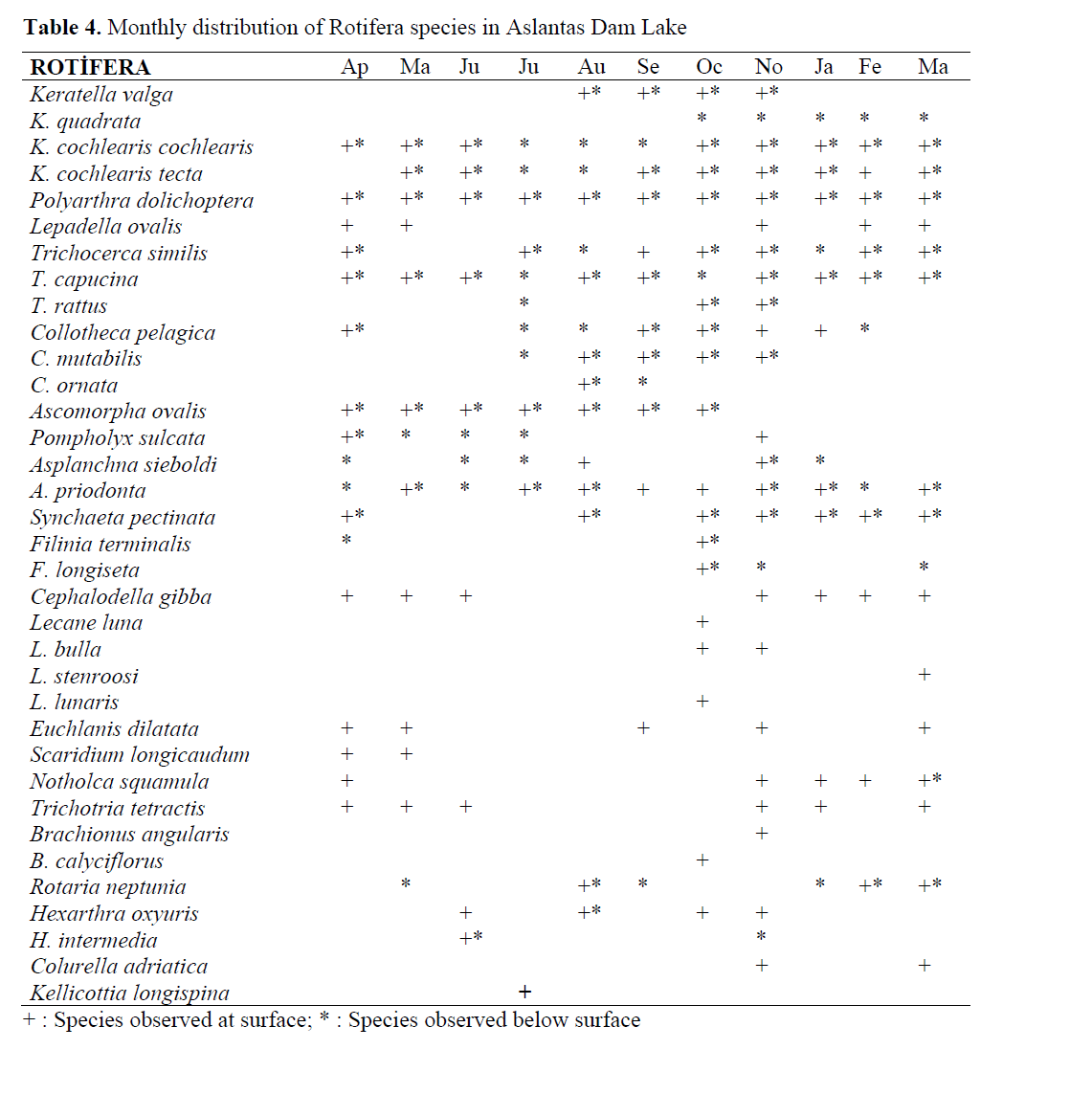
Table 4. Monthly distribution of Rotifera species in Aslantas Dam Lake
Some species such as K. cochlearis cochlearis, Polyarthra dolichoptera, Asplanchna priodonta and Trichocerca capucina were present in the lake throughout the year. Some others such as K. cochlearis tecta, Trichocerca similis, Collotheca pelagica, Synchaeta pectinata, C. gibba were observed most of the year. The rest of the representatives of Rotifera were observed for a period of 6 month or less. It was also determined that some species showed tendency to live at surface, for example, Lepadella ovalis, Lecane luna, L. stenroosi, L. lunaris, L. Bulla, Cephalodella gibba, Euchlanis dilatata, Scaridium longicaudum, Trichotria tetractis, Brachionus angularis, B. calyciflorus, Colurella adriatica and Kellicottia longispina. The species living below the surface only was Keratella quadrata.
The monthly distribution of total Rotifera has been shown in Figure 2. The figure shows that rotifer abundance was the most at surface in August whereas it was March below surface. Rotifers were at the least abundant level in May both surface and below surface.
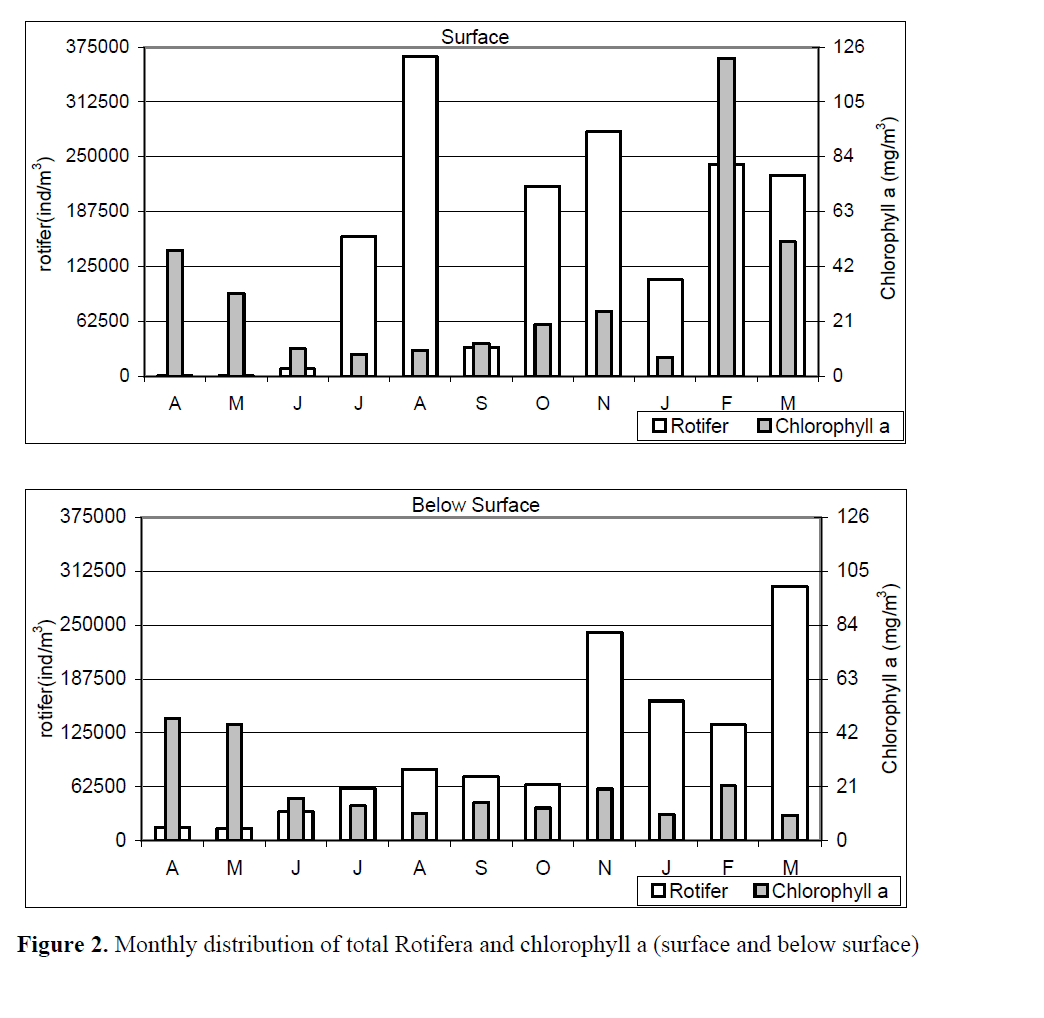
Figure 2. Monthly distribution of total Rotifera and chlorophyll a (surface and below surface)
Vertical distribution
Vertical distribution of total Rotifera has shown in Figure 3. Rotifera were mainly distributed between 2.5 and 10 m. It was found out that the number of total Rotifera increased up to 5 m. There was a constant decrease up to 33 m from 5 m onwards. The number of total Rotifera was the least at 50 m.
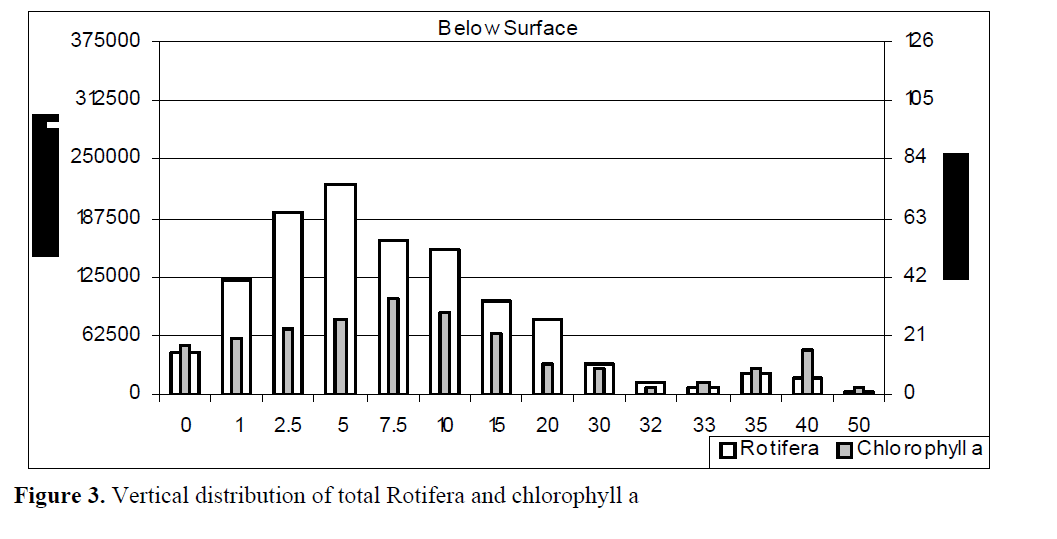
Figure 3. Vertical distribution of total Rotifera and chlorophyll a
Vertical distribution of species is such; the most abundant depth (5 m) occupied mostly by K. cochlearis cochlearis (102458 ind.m-3) and P. dolichoptera (62706 ind.m-3). Main species of the least abundant depth (50 m) were K. cochlearis tecta, T. capucina and Ascomorpha ovalis with a represantation number of 1901 ind m-3, 1098 ind m-3 and 394 ind m-3, respectively.
Light dependent distribution showed that 63.58% of Rotifera prefered to live in photic zone and remaining 36.42% was collected from aphotic zone (Figure 4).
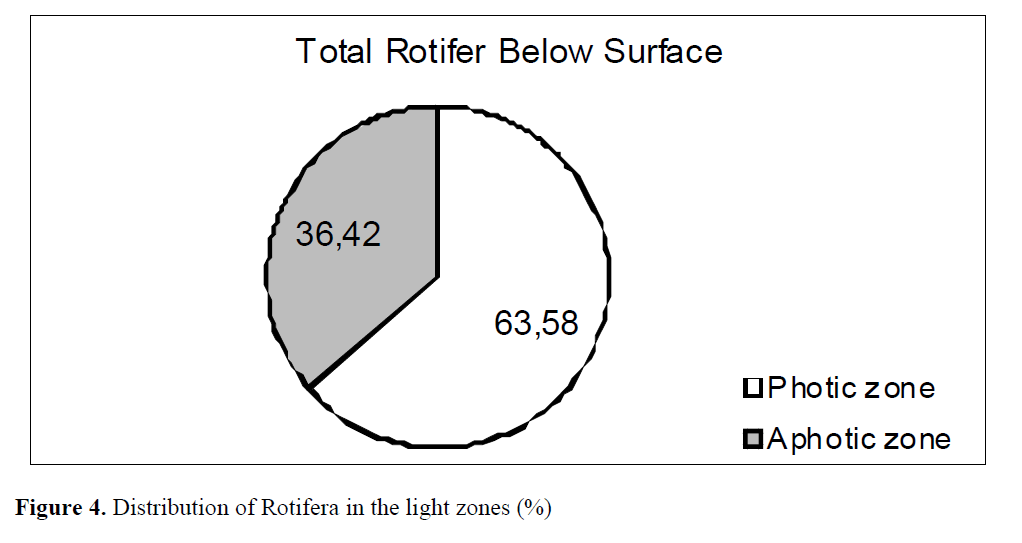
Figure 4. Distribution of Rotifera in the light zones (%)
Vertical Distribution of rotifer Species has shown in Table 5. The most abundant species, K. cochlearis cochlearis (102458 ind m-3) and P. dolichoptera (62706 ind m-3), were commonly found at 5 m depth.
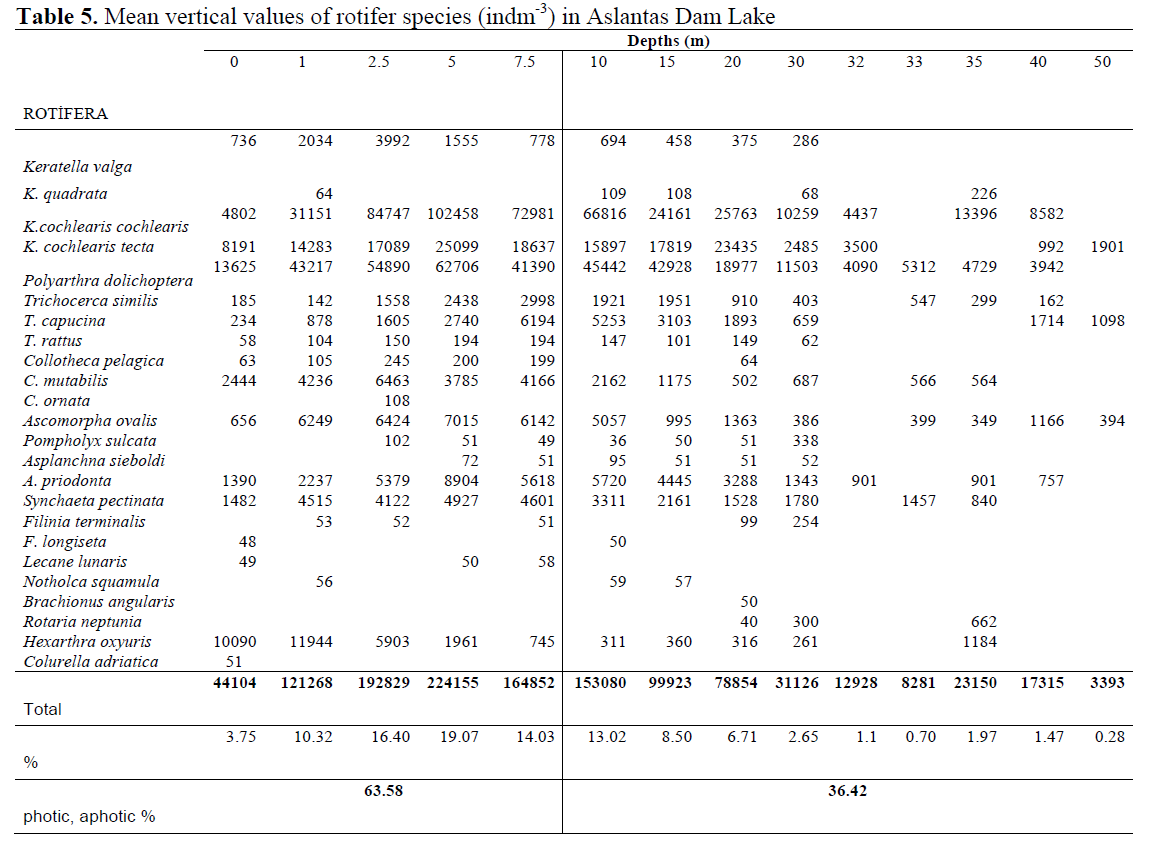
Table 5. Mean vertical values of rotifer species (indm-3) in Aslantas Dam Lake
Analyses revealed that 34.59% of rotifers at surface was P. dolichoptera (603107 ind m-3) and 29.29% of rotifers below surface was K. cochlearis cochlearis (449553 ind m-3). On the other hand the least abundant species at surface was Hexarthra intermedia which represented 0.01% of total Rotifera while there were two species namely B. angularis and C. adriatica, each of which representing only 0.003% of Rotifera below surface.
Rotifer-chlorophyll a interaction
Analyses between rotifer and chlorophyll a interaction monthly and vertically given in Figure 1 and Figure 2, respectively.
While the level of chlorophyll a decreased gradually from April to July at surface and from April to August below surface, a gradual increase at the number of rotifer was observed. Both the number of rotifer and the level of chlorophyll a has fluctuated independently from August onwards (Figure 1).
In consideration of vertical relation between the number of rotifers and the chlorophyll a level, both showed a gradual increase up 5 m. The number of rotifer reached to the highest number at 5 m whereas the level of chlorophyll a was the highest at 7.5 m. Both values reached to the lowest level at 50 m (Figure 2).
As a result of this study a total of 35 species of rotifer has been determined a first record for Aslantas Dam Lake. 33 of them are species and 2 of them are subspecies. Species of Hexarthridae two which determined in Aslantas Dam Lake, is reported to be likely inhabitants of freshwater (Demirhindi, 1972) and they are observed previously, 13 different resoirvuar in Turkey (Demirsoy, 1996). However Hexarthra oxyuris is reported to live in brackish and saline water (Dumont and Ridder, 1987; Ustaoglu and Akyürek, 1994; Brock and Sheel, 1983; Dumont, 1981). These reports show that species of Hexarthridae are mostly euryhaline.
Some of the species i.e., K. longispina, Lecane luna and Notholca squamula inhabits both freshwater and saline habitats. Lepadella ovalis prefers brackish waters and marshlands. Some species occur only a period of year. For example, Rotaria neptunia and B. angularis can be observed only in winter and spring. However, some others, e.g. C. gibba and A. priodonta can be found all year around (Saler and Sen, 2000). It is reported that B. calyciflorus can be found frequently in warm waters; Keratella quadrata lives in all waters as well as oligothrophic and eutrophic lakes (Emir, 1990); Mathew (1979) reports that Pompholyx sulcata is a winter species while Hexarthra intermedia is a hypertermic species. K. longispina is reported to be a hypothermic species (Primicerio and Klemetsen, 1999). This is also supported with the presence of the species in considerably cold water of Turkey such as Abant Lake, Yedigöller and Cip Dam Lake.
The presence of afore mentioned species in Aslantas Dam Lake are related with ecological requirements of these species. However, the presence of L. ovalis, a brackish water and marshland species, all year around and the presence of hypothermic K. longispina in July when Aslantas Dam Lake is not cold at all, is very interesting.
Analyses reveal that the rotifers are distributed abundantly near surface and the total number is the highest at 5 m (Figure 3). Previous reports on the depth dependent distribution of zooplankton claims that rotifers does not show orderly vertical taxis. Instead, most of them prefer proximities close to the surface although they are not likely to be at the surface. However, the number of rotifers decreases as the depth increases (Kolisko, 1974). Nevertheless there are examples of species i.e. Rotaria neptunia, found in depths (Ventela et. al., 1997). Dumont and Ridder (1987) reports that Hexarthra oxyuris shows distribution between 5-20 m we have obtained similar results with R. neptunia which was observed between 20-35 m. H. oxyuris was distributed up to 30 m and they were mostly abundant at 1 m as it is in line with the report of Kolisko (1974).
Kolisko (1974) claims that zooplankton such as rotifers densely, inhabit photic zone where there is an abundance of food as well. Therefore rotifers often do not migrate vertically. Similar findings were obtained as a result of this study. Most of the total Rotifera (63,58%) was collected from photic zone. The remaining 36,42% of Rotifera was obtained from aphotic zone.
Reporters agree that there is a close relation between phytoplankton and zooplankton as far as food chain is concerned. An increase in the number of zooplankton results in a consequential decrease in the number of phytoplankton. The number of zooplankton, usually, increases following the period of phytoplanktonic renewal and development (Cirik and Cirik, 1991; Kolisko, 1974; Horn and Goldman, 1994; Wu and Culver, 1991; Noges, 1997). We have similarly, shown that relatively low number of rotifer was observed while chlorophyll a were increasing gradually. In contrast, a low level of chlorophyll a was determined when the rotifer reached to their peaks. However, a deviation from the reports was observed when the relation between rotifers and chlorophyll a in depths is examined. We found that the number of rotifer decreased when the level of chlorophyll a decreased in depths and vice verse.
Brachionus calyciflorus, B. angularis, K. quadrata, K. cochlearis tecta, Euchlanis dilatata, L. luna, Pompholyx sulcata, Filinia longiseta are reported to be eutrophic species (Rylov, 1963; Borutski, 1963; Brooks, 1971; Voigt and Koste, 1978). However, Kolisko (1974) claims that most of these species inhabits oligotrophic lakes of temperate climates even though they are few in number (200-500 ind L-1). The value of chlorophyll a (31.44 mgm-3) confirms that Aslantas Dam Lake is characteristically not eutrophic.
Conclusions
In conclusion, rotifers in Aslantas Dam Lake are represented with 33 species and 2 subspecies. Some of rotifer species showed tendency to live at surface, for example, Lepadella ovalis, Lecane luna, L. stenroosi, L. lunaris, L. Bulla, Cephalodella gibba, Euchlanis dilatata, Scaridium longicaudum, Trichotria tetractis, Brachionus angularis, B. calyciflorus, Colurella adriatica and Kellicottia longispina. On the other hand, the species living below the surface only was Keratella quadrata. Rotifer species were mainly distributed between 2.5 and 10 m. It was found out that the number of total Rotifera increased up to 5 m. There was a constant decrease up to 33 m from 5 m onwards. The number of total Rotifera was the least at 50 m. Thus, it was found that more rotifer species preferred to live in photic zone (63.58%) and less of them preferred (36.42%) in aphotic zone.
1089
References
- APHA, (1995). Standart Methods for the Examination of Water and Waste Water, 19th Ed. American Public Health Association, Washington, D.C. 1325
- nBerzins, B., (1987). Rotifer Occurrence in Relation to pH, Hydrobiologia, 147: 107-116. doi: 10.1007/BF00025733
- nBorutski, E.V., (1963). Fauna of the USSR Crustacea Vol. III, No. 4 freshwater Harpacticoida. Jorusalem, 314
- nBrock, M.A., Shiel, R.J.,(1983). The composition of aquatic communities in saline wetlands in Avustralia, Hydrobiologia 105(1): 77-84. doi:10.1007/BF00025178
- nBrooks, J.L., (1971). Eutrophication and changes in the composition of the zooplankton. In eutrophication, causes, consequences, correctives. National Academic Science Washington D.C. 236-255
- nCirik, S., Cirik, S., (1991). Limnoloji (Ders Kitabi). Ege Üniversitesi Su Ürünleri Yüksekokulu Yayinlari No: 21, Bornova-Izmir, 135
- nCole, G.A., (1983). Textbook of Limnology. The C.V. Mosby Company, London, 399
- nDemirhindi, Ü.,(1972). Türkiye’nin bazi lagün ve acisu gölleri üzerinde ilk planktonik arastirmalar, Istanbul Üniversitesi Fen Fakültesi Mecmuasi Seri B., 37(3-4): 205-232
- nDemirsoy, A., (1996). Genel ve Türkiye zoocografyasi, hayvan cografyasi. Ankara, 630
- nDSI, (1966). Ceyhan Aslantas Projesi, Asagi Ceyhan Gelistirilmesi Teknik ve Ekonomik Fizibilite Raporu, Adana
- nDSI, (2000). Baliklandirma Çalisma Raporlari. DSI Seyhan Su Ürünleri Arastirma ve Üretim Istasyonu (Basilmamis), Adana
- nDumont, H.J., (1981) , Kratergöl a Deep Hypersaline Crater –Lake in the Steppic Zone of Western Anatolia (Türkey) ,Subject to Occasional Limno-meterological Perturbations, Hydrobiologia, 82: 271-279. doi:10.1007/BF00048721
- nDumont, H.J., Ridder, D. M., (1987). Rotifers from Turkey, Hydrobiologia, 147: 65-73. doi: 10.1007/BF00025727
- nDussart, B., (1969). Les Copepodes des Eaux Continentales d’Europe Occidentale Tale II. Cyclopoides et Biologie. N.Boubee et Cie, Paris, 292
- nEmir, N., (1990). Samsun Bafra Gölü Rotatoria Faunasinin Taksonomik Yönden Incelenmesi, Turkish Journal of Zoology, 14(1): 89- 106
- nHorn, A.J., Goldman, C. R., (1994). Limnoloji. McGraw-Hill, inc. New York 576
- nKolisko, R.A., (1974). Plankton Rotifers Biology and Taxonomy. Biological Station Lunz of the Austrian Academy of Science, Stutgart, 146
- nMathev, P.M., (1979). Studies on the Zooplankton of a Tropical Lake Central Inland Fisheries Fauna. India
- nMikschi, E., (1973). Rotifer Distribution in Relation to Temperature and Oxygen Content, Hydrobiologia, 186/187: 209-214. Moss, B., (1988). Ecology of fresh waters. Man and medium. London: Blackwell Science, 417
- nNoges, T., (1997). Zooplankton-Phytoplankton Interactions in Lakes Vortsjarv, Peipsi (Estonia) and Yaskhan (Turkmenia). Hydrobiologia, Belgium, 342/343: 175-184
- nPrimicerio, R., Klemetsen, A., (1999). Zooplankton Seasonal Dynamics in the Neighbouring Lakes Takvatn and Lombola (Northern Norway), Hydrobiologia, Netherlands, 411: 19-29. doi:10.1023/A:1003823200449
- nRylov, V.M., (1963). Fauna of USSR Crustacea Vol. III, No. 3, Freshwater Cyclopoida. Jorusalem 314
- nSaksena, N. D., (1987).Rotifera as indicators of water quality, Acta Hydrochimica et Hydrobiologica, 15: 481-485. doi:10.1002/aheh.19870150507
- nSaler, S., Sen, D., (2000). Cip Baraj Gölü (Elazig) Rotifera Faunasinin Taksonomik Yönden Incelenmesi, Firat Üniversitesi Fen ve Mühendislik Bilimleri Dergisi, 12(1): 329-337
- nSharma, B. K., (1983).The Indian Species of The Genus Brachionus (Eurotatoria: Monogononta: Brachionida), Hydrobiologia, 104: 31-39
- nStemberger, R.S., (1979). A Guide to Rotifers of the Laurentian Great Lakes, Environmental Monitoring and Support Laboratory Office of Research and Development, U.S. Environmental Protection Agency, EPA-600/4, 185
- nTsalolikhin, S.J., (1994). Key to Freshwater Invertebrates of Russia and adjacent Lands. St Petersburg, 395
- nTsalolikhin, S.J., (1995). Key to Freshwater Invertebrates of Russia and adjacent Lands. St Petersburg, 627 Ustaoglu,M.R., Akyürek, M., (1994). Aksehir Gölü Zooplanktonu. XII. Ulusal Biyoloji Kongresi, Edirne
- nVentela, A. M., Saarikari, V., Vuorio, K., (1997). Vertical and Seasonal Distribution of Micro- Organisms, Zooplankton and Phytoplankton in a Eutrophic Lake. Laboratory of Ecology and Animal Systematics, Hydrobiologia, 363: 229-240. doi:10.1023/A:1003181923569
- nVoigt, M., Koste, W., (1978). Rotatoria. Überordnung Monogononta. Berlin I. Textband, 650, II. Tafelband 234
- nWu, L., Culver, D.A., (1991). Zooplankton Grazing and Phytoplankton Abundance, Journal of Great Lakes Research, 17(4): 425-436.















